CaSO3 Calcium Sulfite Ceramic Balls for Shower Filter Chlorine Removing
Our Advantages:
1.Dust-Free FormulationEliminates dust pollution during handling, ensuring a safer and cleaner working environment.
ETERNAL WORLD CaSO3 Calcium Sulfite Ceramic Balls for Shower Filter Chlorine Removing
Our Advantages:
2.High-Efficiency Chlorine RemovalRapid and complete reaction with chlorine (Cl₂) and hypochlorous acid (HClO), achieving 99% above efficacy with minimal dosage.
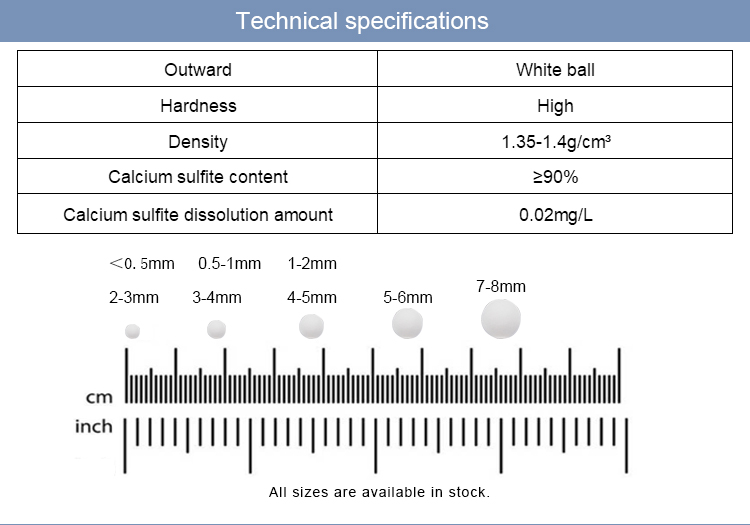
3.Stable & Reliable QualityBatch-to-batch uniformity with strict control over purity (>90%), particle size, and reactivity for predictable performance.
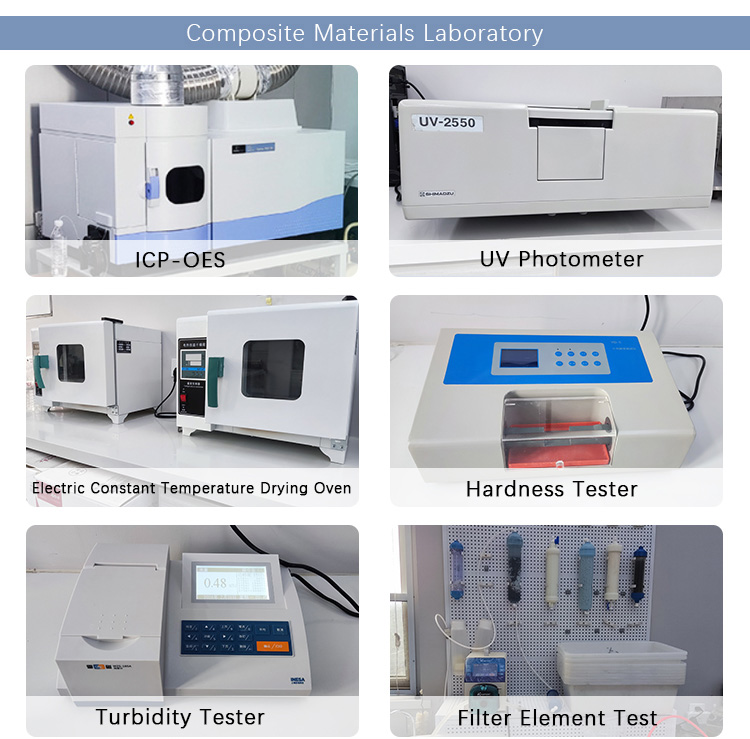
4.Rigorous QC TestingEvery production lot undergoes multi-stage inspections to meet NSF 177 standards.
5.Data-Backed PerformanceTested and verified by our own lab, 3rd party lab tests and customer trials:
i.>99% chlorine removal at 2 ppm residual Cl₂ with flow rate 7L/Min. ii.Long-term stability (shelf life: 50g 4000 liters of water).
| Test Conditions: Filter Media: CaSO3 Ceramic Balls 0.6-1mm Quantity: 40g Test Conditions: (Tap Water) Inlet Chlorine 2ppm. Water Temperature 40℃. Flow Rate: 7.5L/min Test date: Dec. 19th, 2025 | |||
| Filtered Capacity (Litre) | Chlorine before filtration (ppm) | Chlorine after filtration (ppm) | Chlorine Removal Rate |
| 500 | 2.15 | 0.02 | 99% |
| 1000 | 2.04 | 0.02 | 99% |
| 1500 | 2.1 | 0.04 | 98% |
| 2000 | 2.1 | 0.04 | 98% |
| 2500 | 2.02 | 0.04 | 98% |
| 3000 | 1.99 | 0.1 | 95% |
| 3250 | 2.01 | 0.35 | 83% |
| 3550 | 1.98 | 0.59 | 70% |
| 4000 | 2.09 | 0.69 | 67% |
| 4500 | 2.03 | 0.87 | 57% |
Vacuum package.
Brief Introduction:
Chlorine hydrolyzes in water to form hydrochloric acid (HCl) and hypochlorous acid (HClO). Calcium sulfite reduces Cl₂ to chloride ions (Cl⁻) while being oxidized to calcium sulfate (CaSO₄):
CaSO3+Cl2+H2O→CaSO4+2HClCaSO3+Cl2+H2O→CaSO4+2HCl
Ionic Equation:
SO32−+Cl2+H2O→SO42−+2H++2Cl−SO32−+Cl2+H2O→SO42−+2H++2Cl−
Compared to traditional activated carbon for dechlorination, calcium sulfite ceramic balls offer advantages such as long-lasting, high efficient, safe, high-temperature resistance, and no bacterial growth. In comparison with KDF copper-zinc alloy, it has a lower production cost and more effective in removing chlorine.
Add a residual chlorine test reagent to both tap water and water soaked with dechlorination balls. If the water soaked with dechlorination balls does not turn yellow, it indicates that the dechlorination balls can effectively remove the residual chlorine from the water.
Add blue ink reagent to both tap water and water soaked with dechlorination balls. If the water soaked with dechlorination balls does not turn blue, it indicates that the dechlorination balls can effectively remove industrial pollutants from the water.
AS COMPLYING WITH NSF/ANSI 42 AND ALL APPLICABLE REQUIREMENTS PRODUCTS APPEARING IN THE NSF OFFICIAL LISTING ARE AUTHORIZED TO BEAR THE NSF MARK.